is aluminum a good thermal conductor?
When it comes to heat transfer, the choice of materials plays a crucial role. Aluminum, a widely used metal, is often praised for its impressive thermal conductivity. In this article, we will explore whether aluminum truly lives up to its reputation as a good thermal conductor.
Understanding Thermal Conductivity
Before delving into the specifics of aluminum as a thermal conductor, let's first understand what thermal conductivity entails. Thermal conductivity refers to a material's ability to conduct heat. It quantifies how quickly heat can pass through a substance. The higher the thermal conductivity, the better the material is at transferring heat.
The Role of Aluminum in Heat Transfer
Aluminum is renowned for its excellent thermal conductivity. It ranks only behind copper and silver in terms of thermal conductivity among common metals. This property makes aluminum an ideal choice for various applications where efficient heat transfer is crucial.
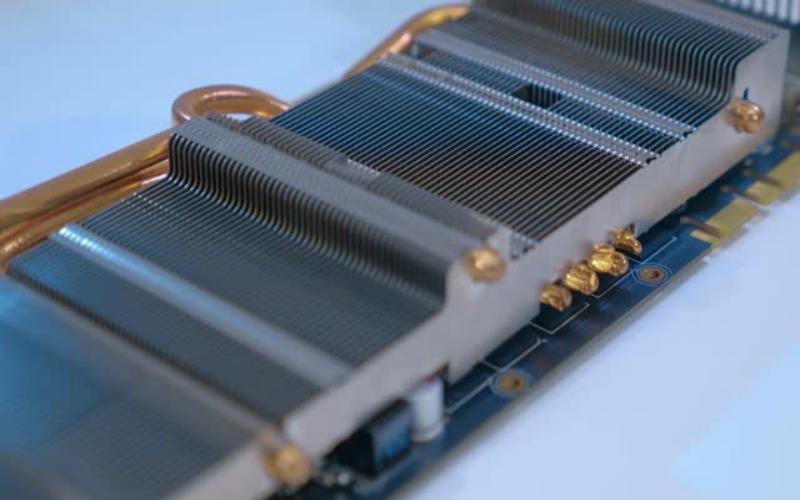
1. Aluminum in Heat Sinks
Heat sinks are commonly used in electronic devices to dissipate excess heat and prevent overheating. Aluminum, with its high thermal conductivity, is frequently employed in the construction of heat sinks. Its ability to efficiently transfer heat away from sensitive components makes it an ideal material for this purpose.
2. Aluminum in Cooking Utensils
When it comes to cookware, aluminum is a popular choice due to its excellent heat conductivity. This property ensures even heat distribution, allowing for precise cooking. Aluminum pans and pots can heat up quickly and maintain a consistent temperature, making them a favorite among chefs and home cooks alike.
3. Aluminum in HVAC Systems
Heating, ventilation, and air conditioning (HVAC) systems rely on efficient heat transfer to maintain comfortable indoor temperatures. Aluminum, with its high thermal conductivity, is often used in HVAC systems to help transfer heat effectively. Whether it's in radiators, heat exchangers, or air conditioning coils, aluminum plays a vital role in ensuring efficient heat transfer.
The Science Behind Aluminum's Thermal Conductivity
Aluminum's exceptional thermal conductivity can be attributed to its atomic structure and the movement of its electrons. The metal has a close-packed hexagonal structure, allowing for better heat transfer. Additionally, aluminum's free electrons move rapidly, facilitating the transfer of thermal energy.
Comparing Aluminum's Thermal Conductivity
While aluminum is widely regarded as a good thermal conductor, how does it compare to other materials? Let's examine its thermal conductivity in relation to a few common substances:
1. Aluminum vs. Copper
Copper is often considered the gold standard for thermal conductivity. It surpasses aluminum in terms of thermal conductivity, making it slightly better at transferring heat. However, aluminum is significantly lighter and more cost-effective than copper, making it a popular alternative in many applications.
2. Aluminum vs. Steel
Compared to steel, aluminum has approximately three times the thermal conductivity. This makes aluminum a better choice when heat transfer efficiency is crucial. Steel may be stronger, but aluminum's superior thermal conductivity outweighs its strength in certain applications.
3. Aluminum vs. Insulators
When it comes to materials with low thermal conductivity, such as insulators, aluminum stands out as an excellent thermal conductor. Its ability to efficiently transfer heat makes it a valuable material in various industries where insulation is not desired.
Conclusion
Aluminum's impressive thermal conductivity makes it a sought-after material in many industries. From heat sinks to cooking utensils and HVAC systems, its ability to efficiently transfer heat has earned it a solid reputation. While it may not surpass copper in terms of thermal conductivity, aluminum's lightweight nature and cost-effectiveness make it an attractive choice for numerous applications. So, if you are in search of a good thermal conductor, aluminum is definitely worth considering.